Organic lab 2 Thin Layer Chromatography. Approximately 80 of the caffeine in the tea leaves can be recovered as crude caffeine.

Flowsheet Of Extraction Of Caffeine From Waste Tea Download Scientific Diagram
This is 0727 pure.

. Here the organic solvent Dichloromethane is used to extract caffeine from aqueous extract of tea powder because caffeine is more soluble in dichloromethane 140mgml than it is in water. Sodium carbonate and varying solvents we are going to extract caffeine from coffee. In order to extract caffeine from tea two bags of tea-leaves each 225g were placed in a 100-mLbeaker containing 50-mL of hot boiling water.
The technique used to separate an organic compound from a mixture of compounds is called Extraction. Ferric reducing antioxidant power FRAP of 010 mM Fe IIg. 10 gm of coffee was boiled for 15-20 mins with sodium carbonate as base.
Extraction of the Caffeine from the Tea. Into the 50 ml Erlenmeyer flask. In this lab Organic Chemistry 1 lab students were able to isolate caffeine from a tea into a powdered solid helping teach the basic principles of extraction and purification.
Caffeine Extraction 3 mL of dichloromethane was added to the centrifuge tube to extract the caffeine from the tea. The resulting aqueous solution was transferred to a separating funnel and 05g of. In-Class Assignment 2 - orgo 2 practice.
Take 500 ml beaker add 200 ml of distilled water to it. Take 5 tea bags and record the weight of these tea bags. Press the tea bags with a clean cork to express as much liquid as possible without breaking the bag and transfer this liquid to the 50 ml Erlenmeyer flask also.
And acid-base liquid-liquid extraction took place in order to force caffeine into organic layer. CHEM 2312 syllabus 1 - Summary Organic Chemistry Ii. Pure product of 0028g of caffeine was obtained from 38501g of impure tea leaves.
Please consult a licensed physician before taking caffeine pills or any. In this method caffeine is extracted from tea. Cool the tea extract to room temperature.
An orthogonal experiment L16 4 5 was applied to optimize the best removal conditions. A 50 mL beaker along with 2 boiling stones was weighed in advance with a total mass of 2756 g during the extraction process. Tea bags are used as the source of caffeine for this experiment.
The selectivity of caffeineEGCG extraction with water was 088 and the selectivity of caffeineEGCG extraction was 024 with ethanol. Extraction of Caffeine from the Tea Solution Pour the tea solution into a 60 mL separatory funnel be sure that the stopcock is closed before you add the tea solution to the separatory funnel. This is why the tea was boiled with water because the caffeine is soluble in it.
The tea was allowed to steep for approximately 10 minutes after which the pockets were squeezed to press the liquid out. Download Solution Pharmacy Mobile App to Get All Uploaded Notes Model Question Papers Answer Papers Online Test and other GPAT Materials - httpsplay. Extract the tea solution with 6 mL of dichloromethane CH 2 Cl.
The amount of caffeine reco. Cap the tubes and gently shake for several seconds carefully vent the tubes to release any pressure buildup by slowly opening the caps. In an experiment using 2 tea bags 110 mg is the expected yield of caffeine to obtain.
The solvents used in the experiment were an aqueous sodium carbonate and dichloromethane DCM. Orgo experiment 4 Separation of components of Analgesic tablet. Anhydrous calcium chloride pellets were used to dry the solution and.
ABSTRACT The objective of the experiment was to extract the caffeine from tea leaves by use of solid-liquid extraction and liquid-liquid extraction. Recap the tubes and shake for about 30 seconds vent. The tube was capped and a very gentle rocking motion was used to carry out the extraction.
Catechins have been extracted and isolated from tea leaves by numerous methods through several steps including. Thus SCCO₂ extraction with water as a cosolvent is suitable for the selective. When the tea has cooled to room temperature add 3 mL of methylene chloride CH 2Cl 2 to each centrifuge tube.
After each rocking motion the tube was vented by opening the cap a little bit to release the pressure. First of all keeping the base as constant ie. To extract caffeine from tea powder using polar - nonpolar solvent extraction technique.
Transfer the tea extract from the 50 ml Erlenmeyer flask to a 125 ml separatory. In the present study we adopt ultrasonic-enhanced supercritical fluid extraction process to remove caffeine from green tea. This step is called as Solid-Liquid Extraction.
The melting point of caffeine. The purpose of this experiment was to perform a liquid-liquid extraction method to extract the caffeine from the tea bags that were provided and then recrystallize the caffeine. The extraction of caffeine from tea leaves is a common organic chemistry experiment.
In the case of Caffeine extraction from tea powder the solubility of caffeine in water is 22mgml at 25C 180mgml at 80C and 670mgml at 100C. The approximate weight of an individual Lipton tea bag is 200 005 g containing 55 mg of caffeine per bag. In Class Assignment 1 - orgo 2 practice.
When the solution was allowed to settle two distinct layers formed. Caffeine is soluble in water at approximately 2 mgml at 25C 180 mgml at 80 C and 670 mgml at 100C. EXAM1 - orgo 2 exam.
The ternary system employs. 2014 extracted the amount of caffeine from used tea leaves of black white green and red tea using dichloromethane. The extraction results using SCCO₂water were compared with conventional liquid solvent extraction using water or ethanol.
The nitrogen present controls solubility. The solution of these dissolved compounds is. Treatment of the tea leaves extraction of catechins from teas into solvents isolation of catechins from other extracted components and drying the preparations to obtain catechin extracts in a powder form.
Now place the 5 tea bags in this beaker. Caffeine is a drug and taking it in pill form can be extremely harmful and addictive. Discard the tea bags.
Low-caffeine or caffeine-removed tea and its products are widely welcomed on market in recent years. Extraction process selectively dissolves one or more of the mixture compounds into a suitable solvent. The type of tea that is used isnt very important as long as it is caffeinated.
A water1-propanolsodium chloride ternary system was found to be a suitable replacement for the more traditional waterorganochlorine solvent systems. The basic property of caffeine comes from the lone pair of electrons found around the nitrogen.

Extraction Isolation Of Caffeine From Tea Leaves Notes

Extraction Of Caffeine From Tea
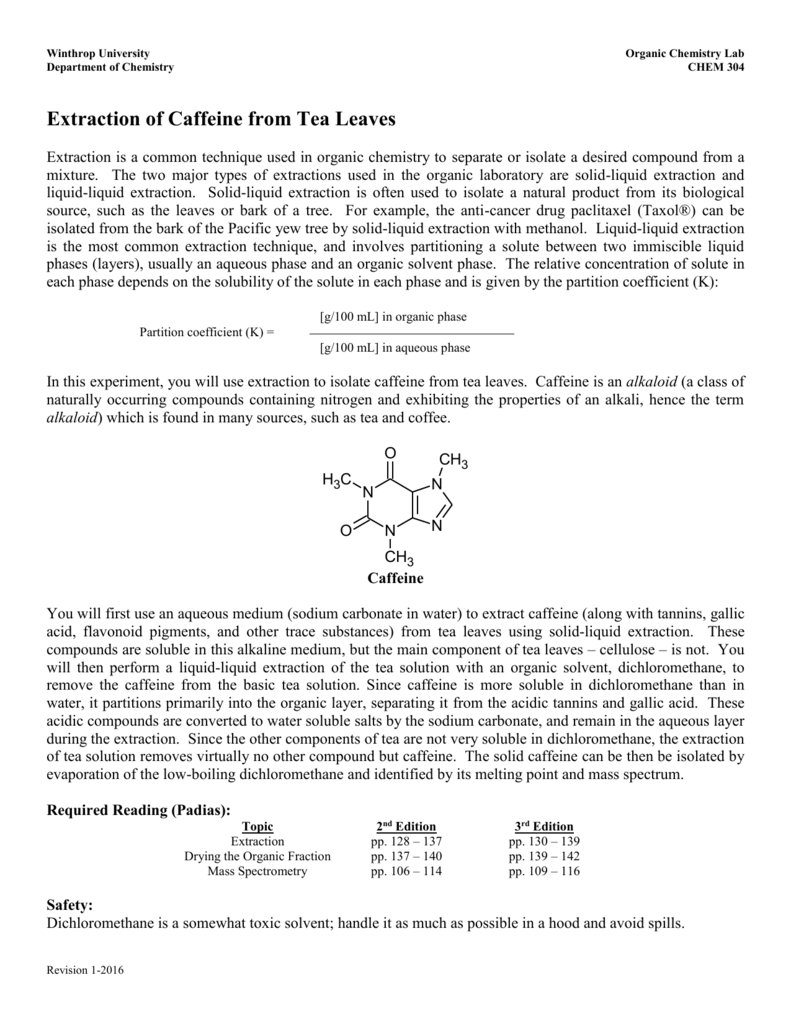
Extraction Of Caffeine From Tea Leaves

Extraction And Isolation Of Caffeine From Tea Leaves English By Solution Pharmacy Youtube

Pdf Isolation And Characterization Of Caffeine From Waste Tea

Pdf The Annals Of Valahia University Of Targoviste 2014 Extraction Of Caffeine From Used Tea Leaves Semantic Scholar
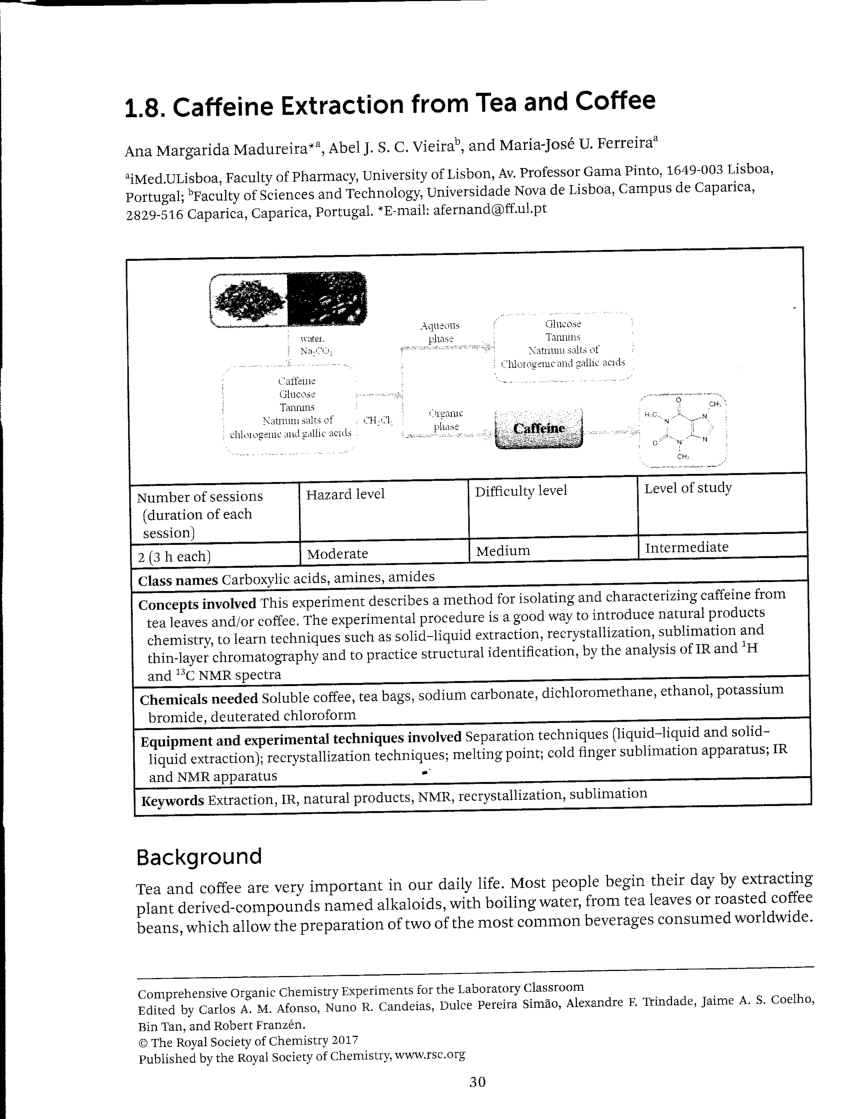
Pdf Caffeine Extraction From Tea And Coffee
Column Chromatography Isolation Of Caffeine From Tea Mendelset
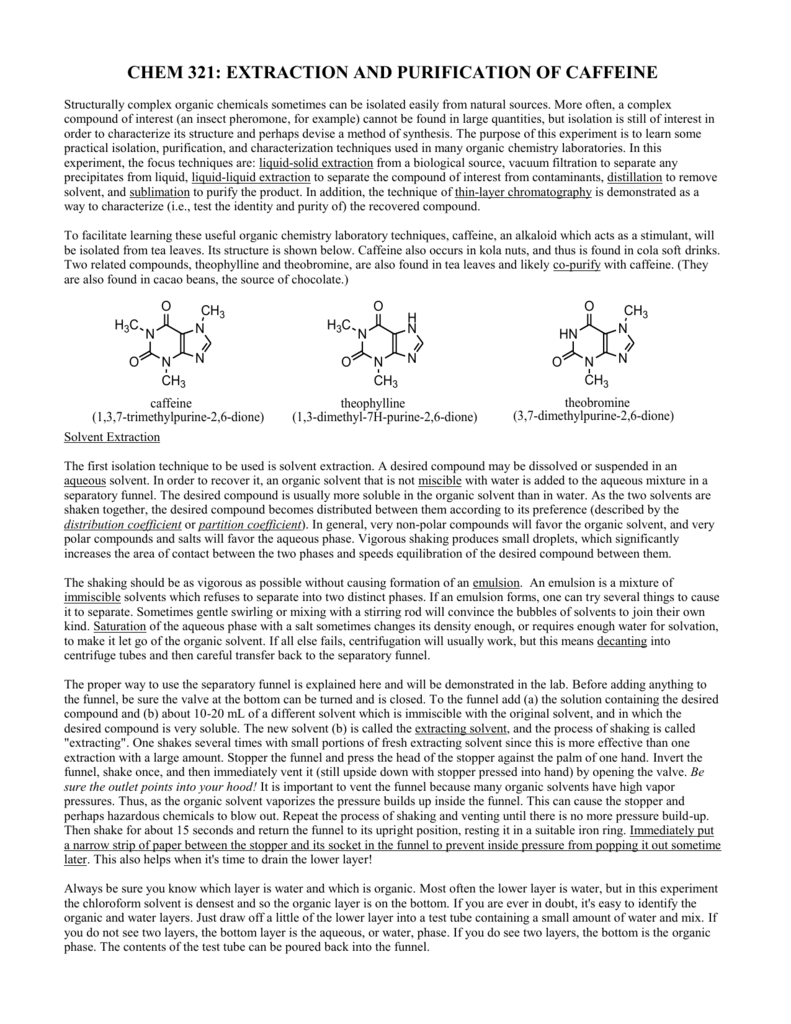
Experiment 2 Isolation Of Caffeine From Tea Leaves

How To Extract Caffeine From Tea Leaves Using A Simple Method I M A 12th Grader Quora

How To Extract Caffeine From Tea Classic Dcm Method Youtube

Process For The Isolation Of Caffeine From Green Tea Color Figure Download Scientific Diagram
Lecture 3 A Extraction Of Caffeine From Tea

Extraction Of Caffeine From Tea Leaves Easy Experiment Dept Of Chemistry Gdc Sopore Youtube

Pdf Experiment 2 Isolation And Sublimation Of Caffeine From Tea Leaves 성철 홍 Academia Edu

Process For The Isolation Of Caffeine From Green Tea Color Figure Download Scientific Diagram
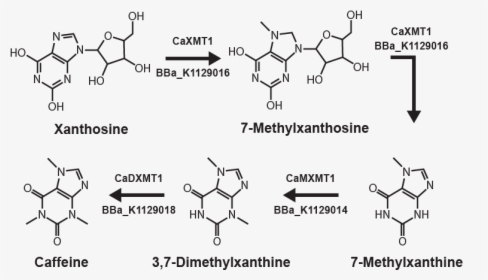
Extraction Of Caffeine From Tea Mechanism Hd Png Download Kindpng
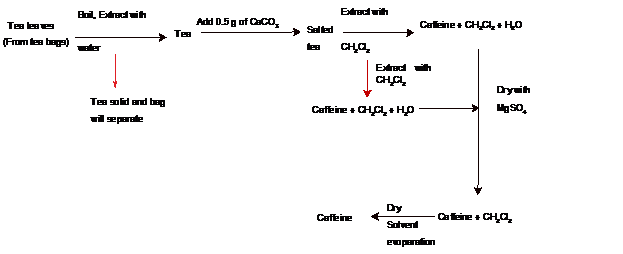
Theoutline Of A Separation Scheme For Isolating Caffeine From Tea Needs To Be Explained Concept Introduction In The Isolation Of Caffeine From Tea Leaves The Main Problem Is That The Caffeine Is

One Part Of Chemistry Extraction Of Caffeine From Tea Leaves
